 Loading... Please wait...
Loading... Please wait...Avoid Battery Sulfation

Several charger companies claim that battery sulfation will not develop if a battery is always kept fully charged. This is incorrect.
What is Battery Sulfation?
Sulfation, a build-up of lead sulfate crystals, is the number one cause of early failures of lead-acid, sealed AGM or flooded (wet cell-filler caps) batteries. A sulfated battery can lead to:
- loss of cranking power
- longer charging times
- excessive heat build-up leading to "boil out"
- shorter running times between charges
- dramatically shorter battery life
Other causes of battery failure include: vibration, contamination, damaged charging plates (due to overheating) and under or over charging.
What Causes a Sulfated Battery?
All lead-acid storage batteries will develop sulfate during their life time. This includes the new sealed "dry" such as Optima, Odyssey, Exide and Interstate branded AGM-spiral-wound types. Batteries develop sulfation each time they are used (discharged - recharged). If they are overcharged or undercharged or left discharged, some for even just several days, they will rapidly develop sulfate. Even when a battery is stored fully charged, sulfate will form unless a desulfating battery charger is used. Using or storing batteries in temperatures above 75°F accelerates the rate of self-discharge and increases battery sulfation dramatically. In fact, the discharge rate doubles, as does sulfation, for every 10°F rise above room temperature.
Use a Desulfating Battery Charger to Reverse the Damage
A sulfated battery can be safely restored using high frequency electronic pulses (NOT high voltage). Unlike other pulse type chargers that claim this or similar sounding features, VDC's BatteryMINDers® desulfating battery chargers use a range of high frequencies. This ensures both old and newly formed battery sulfation will be safely dissolved in the shortest possible time. Other pulse type chargers using just one fixed frequency may remove some, but not all, especially long established - hardened sulfate crystals.
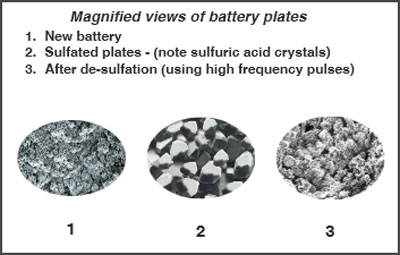
What Makes the BatteryMINDer® Desulfation Method Unique?
Our U.S. Patented methods are truly unique. By generating just the needed range of frequencies and avoiding high voltages, we eliminate potential damage to the batteries storage plates known as "flaking". The sulfuric acid, the major ingredient in a sulfate crystal, can then easily pass into the electrolyte (liquid, gel or absorbed type). This immediately raises its specific gravity and frees the storage plates to now accept a fuller charge. It does this in the shortest possible time, without developing excessive heat. No loss of electrolyte takes place in this process, thus ensuring sealed batteries, as well as "wet", never die due to loss of electrolyte. Testing your battery for sulfation will tell you more about the condition of your battery than any "anecdotal" history ever will.
Getting the Best Battery Performance, Free of Sulfate
As with our own bodies, prevention beats rehab, every time. With BatteryMINDers'® ability to fully charge, without ever overcharging, no matter how long left connected, there is no reason battery sulfation should ever become an issue. Further, without sulfation ever reaching damaging levels and the battery never subjected to overcharging, life and performance can be expected to be several times better than any battery left to self-discharge.
Here are the questions you should be asking yourself:
- Do I want my batteries to last as long as possible (5+ years)?
- Do I want the highest level of performance during their lifetime?
- Do I want to them to charge as fast as safely possible?
- Do I want to eliminate or greatly reduce battery maintenance (adding water, etc.)?
- Do I want to use the lowest amount of electricity to charge my batteries?
- Do I want to win back the price of the charger before I need to replace the battery I first bought it for?1
If you answered yes to any of these questions, then you need a desulfating battery charger. Remember, BatteryMINDers® are backed with a 100% money back guarantee plus a five-year "no hassle", 100% parts and labor or full replacement warranty.
1 Assumes you started with a new battery costing $85 - $150 and that you used the BatteryMINDer® as instructed.
